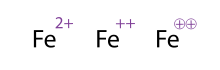When people talk about nuclear power safety they often don't explain certain basic information that you need to know in order to really understand the the subject. This is probably for the best because repeating the same information over and over again would grow old really fast, but unfortunately this leaves people new to the subject unable to fully understand the arguments. The goal of this article is to hopefully be helpful to anyone who doesn't understand the basics. Basically I tried to write something that I think would have been helpful to me when I was starting out.
The Very Basic
 |
| Lithium atom model |
Lets start with the very basic, all the stuff on earth is made up of tiny building blocks called
atoms (
you can see all types on the periodic table of the elements). Atoms are made up of three things. Those three things are
electrons,
protons and
neutrons. Protons and neutron exist in the middle of the atom clumped together in
what is called the nucleus. The electrons exist around that. Atoms can form molecules (i.e. groups of atoms) by sharing electrons.
The
type of atom (i.e. the
element)
is determined by the number of protons in it's nucleus. For example
atoms that have one proton are hydrogen atoms, and atoms that have 92
protons are uranium atoms.
Unlike protons and neutrons, the number of
electrons an atom has can change fairly easily. The default position for atoms is having the same number of electrons as protons. When atoms don't have an equal numbers of protons and electrons they
are called
ions. Knowing what ion you're dealing is important because different ions (even of the same element) behave very differently chemically which is why they came up with Equivalent notations for writing it down.
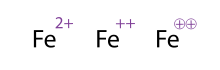 |
| Example of Equivalent Notations |
Every proton is +1, and every electron is -1, simple subtraction tells you what ion you've got. For example iron (Symbol Fe) has 26 protons. If an atom of Iron has 24 electrons then its ionic state is 2+ (26 - 24 = +2), and if it has 28 electrons its ionic state 2- (26 - 28 = -2).
The number of neutrons an atom has determines what type of
isotope
it is. All atoms are some type of isotope even though it's not usually
that important because different isotopes of the same element
(i.e. type of atom) behave the same chemically for most intents
purposes and thus it is often not mentioned or thought about.
Isotopes
are identified by their atomic mass. The atomic mass includes both
protons and neutrons (electrons are very light so they don't count). So for example there are three naturally
occurring isotopes of carbon on earth. They are called carbon-12, carbon-13 and
Carbon-14. Carbon has 6 protons so Carbon-12 has 6 neutrons (12 - 6 = 6), Carbon-13 has 7 neutrons (13 - 6 = 7) and Carbon-14 has has 8 neutrons (14 - 6 = 8).
Ionizing Radiation and Radioisotopes
Not all isotopes are stable. Unstable isotopes eventually decay into different types of atoms releasing
radiation in the process. For example Carbon-12 and Carbon-13 are stable while Cabron-14 eventually decays into
Nitrogen-14 (which is stable).
The unstable isotopes are called
radioisotopes (also known as radionuclide, radioactive nuclide, or radioactive isotopes), and the radiation released is know as
ionizing radiation although most people just call it radiation.
When an unstable isotope decays is random while the probability of it
decaying over any period time is fixed. This probability is understood through something called a half-life. A half-life is the time it takes for half of a given amount of a radioisotope to transmute (i.e. decay) into something else
It's a bit like rolling a dice. Every time you roll a dice the chance of getting a one is the same. If you replace "time you roll a dice" with "fixed period of time" and "getting a one" with "a type radioisotope decaying" it's exactly the same.
You can also use the dice analogy to understand half-lives. Picture that you were rolling a group of six sided dice. Every time you rolled them you remove any dice that lands on a one. The half life of these dice would be three rolls because after three rolls half the dice should be gone. You might be thinking to yourself that half lives aren't very precise because of the random element, but you have to remember that atoms come in large numbers. There are something like 78,000,000,000,000,000,000 atoms in a
grain of sand. If you rolled six dice then at the end maybe half
would be gone or maybe not, but if you are rolling trillions of dice pretty
darn close to half of them would be gone.
In fact half lives are so precise that people use it for dating stuff. There is something called
Radiocarbon dating that uses the half life of Carbon-14 in order to tell how old things are. Carbon-14 is constantly being created in the earth's atmosphere by nitrogen being bombarded by
cosmic radiation. Because it's being created at a constant rate it's also being absorbed by plants at a constant rate and from plants it moves to animals. When something dies it stop taking in Carbon-14 so by using it's half life researches can tell how long ago something died based off the amount of carbon-14 left in it's remains.
Some radioisotopes decay into other radioisotopes. When that happens you have what is called a
decay chain. The decay chain is used to describe how radioisotopes decay until they eventually reach a stable state.
Here is the decay chain for
Thorium a common naturally occurring radioisotope.
You may be wondering at this point where all the radioisotopes on earth come from. Well some
(34 types) are primordial (i.e. they came about before the earth was formed). This includes Uranium, Thorium and Potassium-40.
Some of them are caused by cosmic radiation such as Carbon-14. A small minority are created as a result of human activity, the most common of these activities involves the breaking down of larger radioisotopes into smaller radioisotopes in order to produce energy.
Here is a really good link about radioactivity in the environment if you're interested in learning more about it.
Types of Radiation
The types of radiation produced by radioisotopes include both
Electromagnetic radiation and
Particle radiation. Electromagnetic radiation plays a big part in our lives.
Depending on the frequency it has many different applications and also names. The most familiar form is the visitable spectrum, or more commonly just called light. It's also useful for microwaves ovens, cell phones, radio, x-rays etc.
But for our purposes we are only interested in electromagnetic radiation that is ionizing radiation. Ionizing radiation is radiation that has enough energy to knock electrons off atoms (or molecules) thus ionizing them. This is important because as we talked about before different ions behave much differently chemically. This can cause problems. In most cases a few atoms (or molecules) being ionized doesn't matter much, but in some cases it does. For example ionizing radiation can cause harm to living tissue. You've probably noticed such harm yourself if you've ever spent too much time in the sun and
got sunburned.
Some atoms (and molecules) hold their electrons better than others so what types of radiation are ionizing isn't so clear cut, but really what we care most about is the effect of ionizing on human beings so I would say ultraviolet (sun burn) rang and higher (higher frequency that is, the higher the frequency the more energetic the more able to knock of electrons) is ionizing radiation.
Usually when we are talking about radioisotopes decaying we are talking about
gamma rays (i.e. y-rays), not X-rays or ultraviolet. Lower wave length ionizing radiation can be created as secondary radiation (i.e. ionizing radiation created by other ionizing radiation) though.
Particle radiation is simply particles that are moving very quickly.
The two types of particles that matter for what we are talking about
are
Alpha particles and
Beta particles.
Alpha particles are helium-4 atoms without any electron. The ones created by radioactive decay have a strong ability to ionize things, but they have little ability to penetrate shielding and can be stopped by a piece of paper or the thin layer of dead skin all of us have.
Beta particles are electrons or sometimes positrons. Positrons are the antimatter equivalent of electrons. When electrons and positrons meat they destroy each other releasing some gamma rays in the process.
Metric Prefixes
Now that we've covered what radiation is and where it comes from lets talk about how it's measured. Well before that we have to talk about something call
metric prefixes. If you spend time reading about this subject you're going to encounter these things a lot.
 |
| For Micro it's usually abbreviated μ or mc |
Metric prefixes are used for writing really large or really small numbers without having to write all the zeros. It's very similar to
scientific notations in that regards. The prefix goes before the unit abbreviation. For example with 10 cm the c is the metric prefix (centi) and the m is the abbreviated unit types (meter).
It's fairly easy to convert a number to a different prefix or know the number in it's entirety (i.e. what the number is without prefixes). Look at the table above to the left of prefix you want to convert from. The number to the right of the 10 is the one we're interested in. Take that number and subtract the number next to the 10 of the prefix you want to convert to. If you want to convert to no prefixes then subtract zero. If the number you get is negative move the decimal point that many spaces to left, if it's positive move the decimal point that many places to the right.
For example 1,000 nm (-9 - (-6) = -3) = 1 µm , 1,000 µm (-6 - (-3) = -3) = 1 mm, 1,000 mm (-3 - 0 = -3) = 1 m, and 1,000 m (0 - 3 = -3 ) = 1 km. Where m stands for meters.
Measuring Radiation
There are a lot of different ways of measuring radiation. I'll try and go over the most common ones.
Activity (A)
Activity measures the number of nucleus decays. It's calculated using amounts of radioisotopes and knowledge of there half lives. The two main units for this are Becquerel, and Curie.
The
becquerel (symbol Bq) it is the
SI derived unit of radioactivity. One Bq is defined as the activity of a quantity of radioactive material (i.e. radioisotopes) in which one nucleus decays per second. Basically it's a unit used to describe the amount of radiation produced by some amount of radioisotopes.
The
curie (symbol Ci) is a non-SI unit of radioactivity, named after Marie and Pierre Curie. It is defined as 1 Ci = 3.7 × 10
10 decays per second.
Conversion factors:
- 1 GBq = 0.027 Ci
Absorbed dose (D)
Absorbed dose measures the energy (from ionizing radiation) absorbed by a mass. This is important because it takes energy to ionize things so knowing how much energy is going towards ionizing things help you know how much stuff is getting ionized. This in turn can help give you some idea of the effect. Most
Geiger counters measure this. The two main unit types for this are Rad and Grey.
The SI unit for absorbed dose is the
gray (Gy). One gray is the absorption of one joule of energy, in the form of ionizing radiation, per kilogram of matter.
The other unit is called the
Rad. The rad is a non-SI CGS unit that is sometimes also used, predominantly in the USA.
Conversion factor:
- 1 rad = 0.01 Gy
Dose equivalent (H)
Dose equivalent is a measure of the health effect of low levels of ionizing radiation on the human body. The two main unit types for this are Roentgen and Sievert. Quantities that are measured in roentgens or sieverts are intended to represent the stochastic health risk, which for radiation dose assessment is defined as the probability of cancer induction and genetic damage. There is no way to directly measure equivalent dose. Instead other measurements are used to arrive at equivalent dose using various conventions. For example with X-rays and gamma rays the gray is numerically the same value when expressed as the
sievert (Sv), but for
alpha particles one gray is equivalent to twenty
sieverts because of the radiation weighting factor that is applied.
Conversion factor:
- 1 rem = 0.01 Sv
Radiation Inside the Body
Internal doses can be worse than external one's (depends on amounts and other factors). For example alpha particles can be stopped by a thin layer of dead skin making them fairly harmless outside the body, but more dangerous than other types of radiation inside it. In fact a large part of people's average annual doses comes from alpha particles produced by the decay of
Radon (symbol Rn). Radon is an odourless, colourless, gas that exists in small amounts all around us. Radon is constantly being created as part of the decay chain of all the naturally occurring isotopes of uranium and thorium.
At any rate predicting internal doses is important. An important thing to remember when internal doses are concerned is that different isotopes behave the same chemically. This can be both a good thing and a bad thing. For example
Iodine-131 is a radioactive isotope of iodine that is produced a lot in nuclear reactors. It has a half life of about 8 days. Like all iodine it's utilized by the thyroid which means if it gets released into the environment it can be a problem. Luckily by taking potassium-iodide pill you can flood your body with non radioactive iodine so Iodine-131 wont get absorbed, and because it's half life is so short it will be gone in short order. Because they behave the same chemically radioisotopes also have many beneficial uses such as
Radiopharmacology a branch of medicine which uses radioisotopes for medical imaging and in therapy for many diseases (for example, brachytherapy). Ironically Iodine-131 is also one of the radioisotope used in medicine.
Internal Dosimetry
Internal dosimetry is the science and art of internal ionizing radiation dose assessment due to radioisotopes incorporated inside the human body. Radioisotopes deposited within a body will irradiate tissues and organs and give rise to
committed dose until they are excreted from the body or the radionuclide is completely decayed. The internal doses for workers or members of the public exposed to the
intake of radioactive particulates can be estimated using
bioassay data such as lung and body counter measurements, urine or faecal radioisotope concentration, etc.
Man-Made Radiation Exposure Breakdown
This kind of depends on what you think of as man made exposure. For example is Radon pumped into people's houses along with natural gas man made or natural exposure? At any rate, not counting stuff like Radon most man made radiation exposure is a result of various medical procedures (such as x-ray). This accounts for around 20% of exposure worldwide and up to 50% of exposure in industrialized countries.
Here is a pie chart.
Harm
Linear no threshold model
The most widely accepted model for determining harm for low doses is known as the
Linear no threshold model (LNT). For this model it doesn't matter how much radiation you
receive. All radiation can cause harm, all radiation has an equal
chance of causing harm.
Here is a nifty online calculator for it applying it.
Organizations That Support LNT
United States National Research Council
"The assumption that any stimulatory hormetic effects from low doses of
ionizing radiation will have a significant health benefit to humans that
exceeds potential detrimental effects from the radiation exposure is
unwarranted at this time."
United States National Academies
"The scientific research base shows that there is no threshold of
exposure below which low levels of ionizing radiation can be
demonstrated to be harmless or beneficial."
National Council on Radiation Protection and Measurements
United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR)
Until the [...] uncertainties on low-dose response are resolved, the
Committee believes that an increase in the risk of tumour induction
proportionate to the radiation dose is consistent with developing
knowledge and that it remains, accordingly, the most scientifically
defensible approximation of low-dose response. However, a strictly
linear dose response should not be expected in all circumstances
The Controversy
Radiation can be harmful. Everyone seems to agree with that. What people can't always agree about is the effect of very small doses of radiation. Anyway
Here is a page that does a good job of describing the controversy. If you want to understand this subject better
this is worth reading.
Living things evolved in a world full of radiation. Various biological defence mechanisms have come about in order to protect organisms from it and other sources of harm.
Here is a list of some of our bodies defences.
- Defences against the metabolically induced reactive oxygen species (i.e. defence against things that have been ionized),
- DNA repair, and
- Elimination of damaged cells.
The big disagreement is low levels of radiation, where it is difficult to show statistically what is going on. There are several different models that describe the effects of radiation at low doses.
Another model is the threshold model
This model says that only radiation over a certain dose is harmful.
Organizations that support this model
French Academy of Sciences (
Académie des Sciences) and the National Academy of Medicine (
Académie nationale de Médecine).
In conclusion, this report raises doubts on the validity of using LNT
for evaluating the carcinogenic risk of low doses (< 100 mSv) and
even more for very low doses (< 10 mSv). The LNT concept can be a
useful pragmatic tool for assessing rules in radioprotection for doses
above 10 mSv; however since it is not based on biological concepts of
our current knowledge, it should not be used without precaution for
assessing by extrapolation the risks associated with low and even more
so, with very low doses (< 10 mSv), especially for benefit-risk
assessments imposed on radiologists by the European directive 97-43.
Hormesis Model
Another model is the is the
hormesis model which postulates that a certain amount of radiation actually decreases your chance of getting cancer a little because it stimulates your body's natural defences.
What People agree About
At any rate pretty much everyone agree about larger doses so
Here are a few facts I think anyone would agree with.
- 100 rem received in a short time can cause observable health
effects from which your body will likely recover, and will increase your
chances of getting cancer.
- 1,000 rem in a short or long period of time will cause
immediately observable health effects and is likely to cause death.
Conclusion
As for conclusions there isn't one really. Hope this was helpful to someone.
Update: I've made a lot of changes in order to make it more complete. Also, changes some things to make it more balanced.